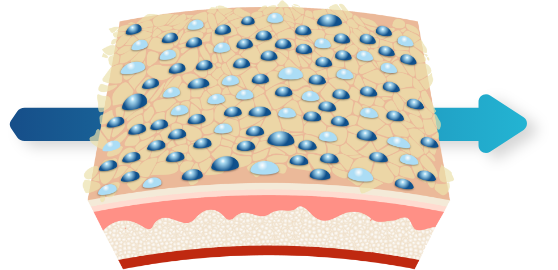
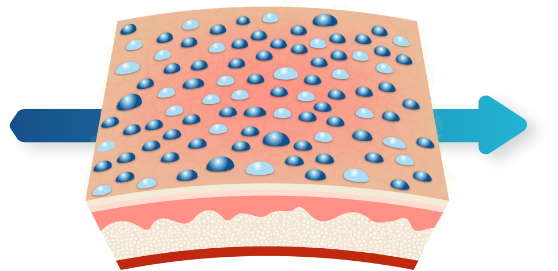
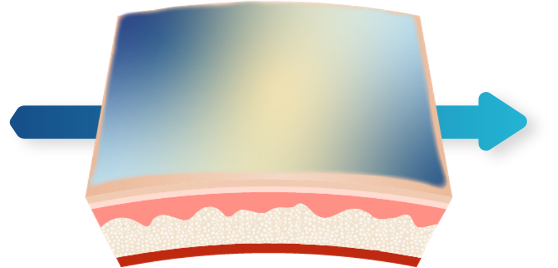
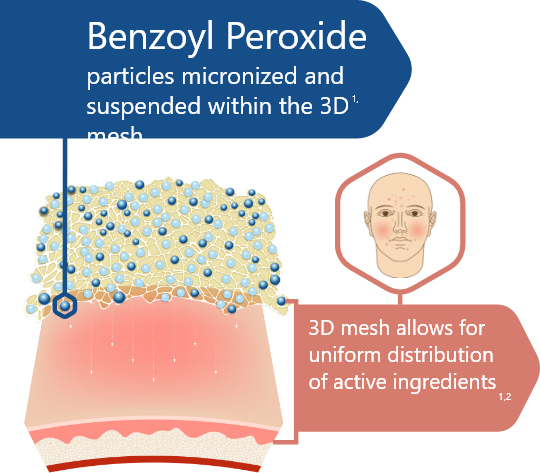
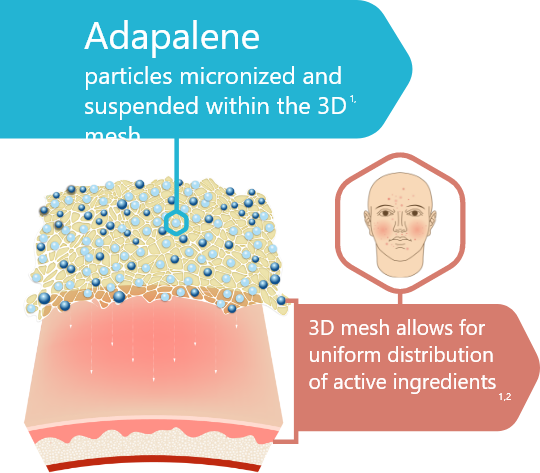
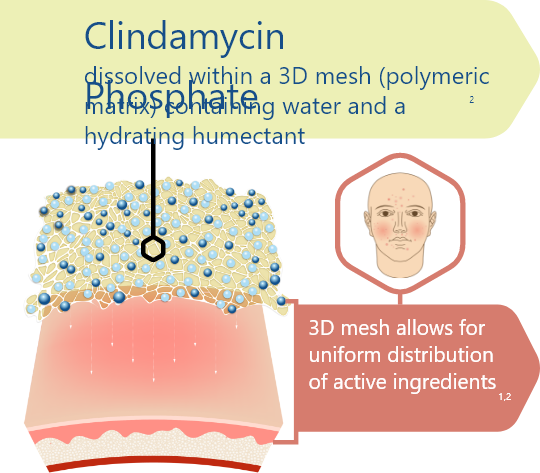
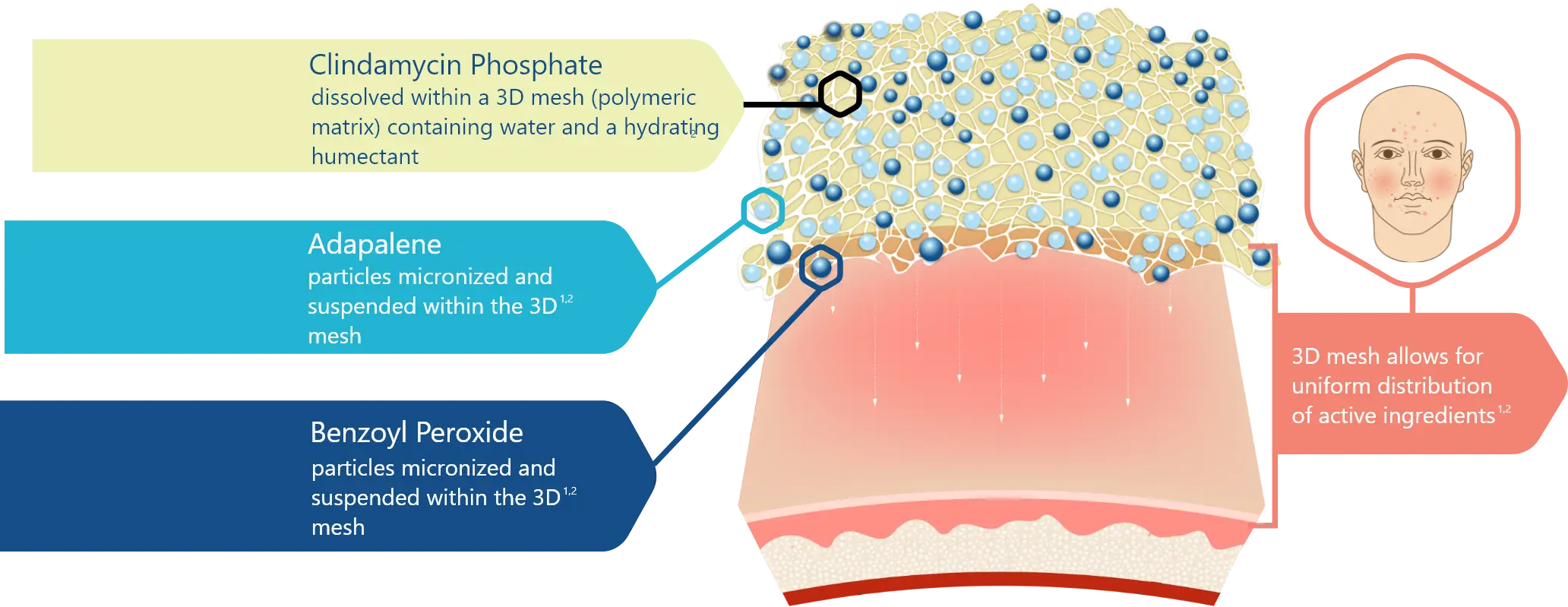
3D Delivery Technology1,2
90% of micronized BPO and adapalene particles are < 10µm in size
Water-based gel designed with only four inactive ingredients1,2:
- Purified water (solvent)
- Carbomer homopolymer (gelling agent)
- Propylene glycol (humectant)
- Potassium hydroxide (alkalizing agent)

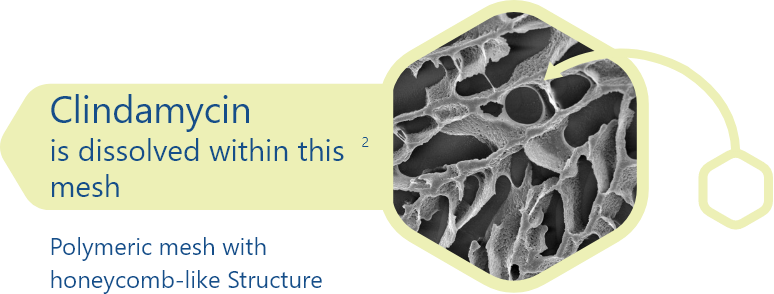



Click below to see more on the polymeric mesh technology
Polymeric mesh collapses instantly upon contact with salts on skin

Polymeric mesh collapses instantly upon contact with salts on skin
Application Reminders for Patients
Ensure your patients read the Instructions for Use before they start using CABTREO and each time before they get a refill.

Patients should wash their face with a gentle, mild cleanser before applying CABTREO.

Apply onto 6 areas of their face.

Dispense one pea-sized amount onto finger.

Patients may use a moisturizer to minimize irritation.
Remind patients to avoid concomitant acne therapies.
ONE SCRIPT. ONE 50G PUMP.
ONE APPLICATION A DAY.1
With one application a day, CABTREO can become a part of your patient’s daily routine.1


Not actual patient.
Important Safety Information AND INDICATION
CONTRAINDICATIONS
CABTREO is contraindicated in patients with:
- known hypersensitivity to clindamycin, adapalene, benzoyl peroxide, any components of the formulation, or lincomycin.
- history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis.
References: 1. Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Amer J Clin Dermatol. 2022;23(1):93-104. 2. Ortho Dermatologics. Data on file. 3. CABTREO (clindamycin phosphate, adapalene and benzoyl peroxide) Topical Gel 1.2%/0.15%/3.1% [prescribing information]. Bridgewater, NJ. Bausch Health US, LLC.